Seminario Vittorio Pace, University of Turin, Italy
Osvaldo Lanzalunga vi invita al seminario:
Designing New Synthetic Concepts with C-1 Sources
di Vittorio Pace, University of Turin, Italy
Il seminario si terrà lunedì 19 febbraio, alle ore 12 in Sala Parravano , primo piano dell'edificio Cannizzaro (CU014).
Abstract
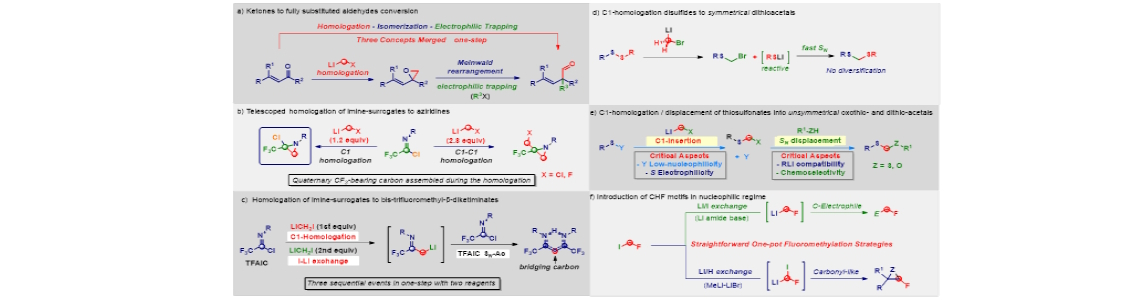
The direct transfer of a reactive nucleophilic CH 2 X unit into an existing linkage enables the formal introduction of the moiety with the precisely defined degree of functionalization.1 Upon the fine tuning of the reaction conditions governing the transformation, the initial homologation event can serve as the manifold for triggering unusual rearrangement sequences leading to complex architectures through a unique synthetic operation. The direct – full chemoselective - conversion of a ketone into the homologated all-carbon quaternary aldehyde (via a) 2 , the telescoped homologation of imine-surrogates to quaternary aziridines (via b) 3 and bis-trifluoromethyl-β-diketiminates (via c) will illustrate these unprecedented concepts. Additionally, the homologation of disulfides and thiosulfonates will furnish symmetrical (via d) and unsymmetrical oxothio- and dithio-acetals (via e). The one-step mono-fluoromethylation of carbon electrophiles with extremely labile fluoromethyllithium reagents will provide a novel entry to valuable fluorinated building-blocks without the needing of using protecting elements for fluoro-containing carbanions (via f). 4 Finally, the development of homologation strategies not relying on the use of external C1-sources will be discussed. 5
References
(1) (a) Castoldi, L.; Monticelli, S.; Senatore, R.; Ielo, L.; Pace, V. Chem. Commun. 2018, 54, 6692-6704. (b) Senatore, R.; Castoldi, L.; Ielo, L.; Holzer, W.; Pace, V. Org. Lett. 2018, 20, 2685-2688. Homologation Reactions. Reagents, Applications and Mechanisms. (Pace, V. Ed.) Wiley-VCH, Weinheim 2022, in press (ISBN: 978-3-527-34815-2).
(2) Pace, V.; Castoldi, L.; Mazzeo, E.; Rui, M.; Langer, T.; Holzer, W. Angew. Chem. Int. Ed. 2017, 56, 12677-12682.
(3) (a) Ielo, L.; Touqeer, S.; Roller, A.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2019, 58, 2479-2484. (b) Ielo, L.; Castoldi, L.; Touqeer, S.; Lombino, J.; Roller, A.; Prandi, C.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2020, 59, 20852-20857. (c) Senatore, R.; Malik, M.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem. Int. Ed. 2021, 60, 24854- 24858.
(4) (a) Parisi, G.; Colella, M.; Monticelli, S.; Romanazzi, G.; Holzer, W.; Langer, T.; Degennaro, L.; Pace, V.; Luisi, R. J. Am. Chem. Soc. 2017, 139, 13648-13651. (b) Monticelli, S.; Colella, M.; Pillari, V.; Tota, A.; Langer, T.; Holzer, W.; Degennaro, L.; Luisi, R.; Pace, V. Org. Lett. 2019, 21, 584-588. (c) Senatore, R.; Malik, M.; Spreitzer, M.; Holzer, W.; Pace, V. Org. Lett. 2020, 22, 1345-1349. (d) For sequential nucleophilic additions - deoxygenations, see: Miele, M.; Citarella, A.; Langer, T.; Urban, E.; Zehl, M.; Holzer, W.; Ielo, L.; Pace, V. Org. Lett. 2020, 22, 7629-7634.
(5) Malik, M.; Senatore, R.; Langer, T.; Holzer, W.; Pace, V. Chem. Sci. 2023, 14, 10140-10146.
Prof. Dr. VITTORIO PACE Short Bio 
Vittorio Pace (born 1981) graduated in Pharmacy in 2005 from the University of Perugia (Italy) and subsequently, received the PhD in Chemical Sciences cum laude from the Complutense University of Madrid in 2010 working with Profs. Alcántara and Sinisterra. After postdoctoral training at Vienna (Prof. Holzer, 2010-2011), Manchester (Prof. Procter, 2011-2013) and Stockholm (Prof. Olofsson, 2013-2014), he obtained a group leader position at the University of Vienna in 2014, before holding the Tenure-Track Professorship in Drug Synthesis at the University of Vienna between 2018 and 2020. Since March 2020 he is Full Professor of Organic Chemistry at the University of Torino. In 2016 he received the Habilitation in Pharmaceutical Chemistry from the University of Vienna and, in 2017 the Habilitation for Full Professor of Organic Chemistry from the Italian Ministry of University. He received several awards including the Ciamician Medal of the Italian Chemical Society, the Caglioti Prize of the Accademia Nazionale dei Lincei, the Young Investigator Award of the Faculty of Life Sciences at Vienna, the La Roche-Hoffmann Prize of the European Society of Medicinal Chemistry, the Viennese Innitzer Award in 2017, the Habilitation Award of the Austrian Chemical Society in 2019 and the Thieme Journal Award in 2020. Hisresearch core is represented by the design and development of new synthetic concepts with functionalized organometallic reagents.
