Pot Economical Synthesis of Biologically Active Molecules using Organocatalyst
Fabrizio Vetica è lieto di invitarvi al seminario
Pot Economical Synthesis of Biologically Active Molecules using Organocatalyst
di Yujiro Hayashi, Tohoku University, Sendai, Giappone
ospite del Dipartimento di Chimica, che si terrà in sala Parravano, piano I edificio S. Cannizzaro CU014, martedì 27 settembre alle ore 11.00 (link piattaforma Google Meet: axp-tjue-pjv)
Abstract
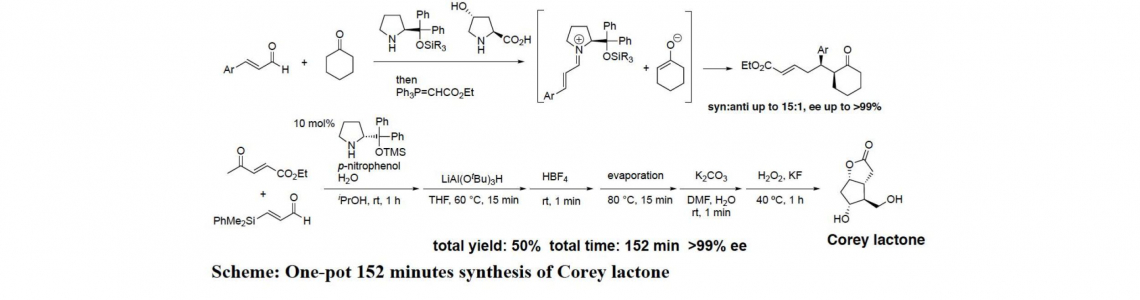
One-pot operations are an effective method for both carrying out several transformations and forming several bonds in a single-pot, while at the same time cutting out several purifications, minimizing chemical waste generation, and saving time. Thus, a one-pot reaction can be not only efficient, but also green and environmentally friendly, and “pot-economy” should be considered in planning a synthesis.On the other hand, organocatalyst is an effective catalyst to carry out several reactions in a same vessel. Our group and Jørgensen’s group independently discovered that diphenylprolinol silyl ether is an effective organocatalyst in the reaction involving enamine and iminium ion as reactive intermediates. We have been investigating the application of this organocatalyst to the one-pot synthesis of biologically active molecules.We have accomplished “one-pot” and 152 minutes total synthesis of Corey lactone, in which a key reaction is a Michael reaction of ketone and a,b-unsaturated aldehyde. We also accomplished a five pot synthesis of (–)-quinine. In the presentation, not only the syntheses of Corey lactone and quinine, but also the reaction mechanism of asymmetric Michael reaction of ketone and a,b-unsaturated aldehyde catalyzed by similar two amine catalysts such as diphenylprolinol silyl ether and 4-hydroxyproline will be described.
 Yujiro Hayashi
Yujiro Hayashi
The Hayashi laboratory develops and applies new approaches based on synthetic organic chemistry to chemical and biological discovery. Over the past several years we investigated two new lines of research. The first aim is to synthesize biologically important natural products. Total synthesis of these products enables not only steady supply of rare samples but also accelerates the chemical and biological investigations. Development of compounds with superior biological activity rather than natural products also lies in our goal. The second line seeks to develop new synthetic reactions. The reactions must be efficient and environmentally friendly. Our unique catalyst (yellow one in Figures) have been proved to be general and highly efficient. Moreover, our developed reaction with this catalyst allowed the high-yielding synthesis of the anti-influenza neuraminidase inhibitor (-)-oseltamivir (tamifulu) in "one-pot" sequences. We believe that our research contributes to human health and welfare as well as the scientific progress.
