Richard C. D. Brown seminar
Fabrizio Vetica is pleased to invite you to the seminar:
Total Synthesis of Acetogenins and Lupin Alkaloids
Prof. Richard C. D. Brown
The seminar will be held in Parravano Hall, Cannizzaro building CU014, Tuesday 9 May, at 2.30 PM and it can be also followed remotely on the Google Meet platform link.
Abstract
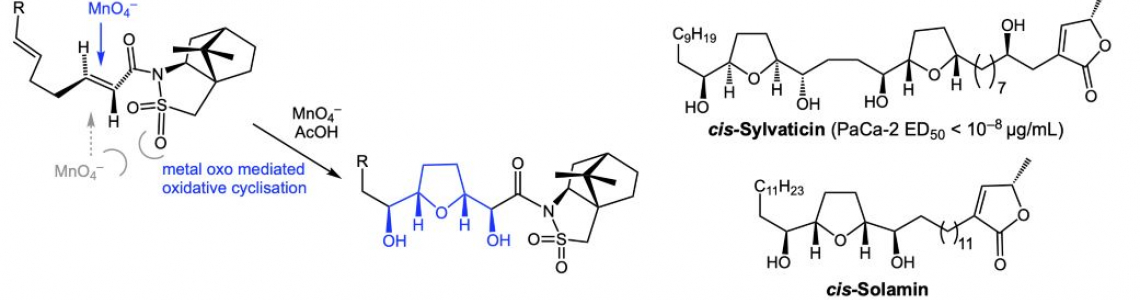
Direct oxidative cyclisation of dienes using metal oxo reagents is one of the most elegant and concise approaches to the synthesis of tetrahydrofuran motifs present in an array of bioactive natural products. Structural and stereochemical complexity is introduced in a single reaction step, including up to four new stereogenic centres and a THF ring. Furthermore, absolute stereochemistry may be controlled through application of chiral auxiliaries,(1) or by chiral phase catalysis in the permanganate-mediated variant of the reaction.(2) This talk will provide an overview of the permanganate oxidative cyclisation reaction and illustrate applications in total synthesis.
The second part of the talk will look at an imino-aldol (or Mannich-type) approach towards lupin alkaloids. By controlling the relative stereochemistry (syn vs anti) in acyclic intermediates, sparteine or b-isosparteine stereoisomers can be synthesised. Recognition of a common quinolizidine motif, present in sparteine and matrine series (based on structurally isomeric skeletons) allows the approach to be applied in a structurally as well as stereodivergent fashion. The total syntheses of alkaloids in the matrine and sparteine will be described.(3)
References:
(1). (a) Morris, C. L.; Hu, Y. L.; Head, G. D.; Brown, L. J.; Whittingham, W. G.; Brown, R. C. D., J. Org. Chem. 2009, 74, 981-988; (b) Brown, L. J.; Spurr, I. B.; Kemp, S. C.; Camp, N. P.; Gibson, K. R.; Brown, R. C. D., Org. Lett. 2008, 10, 2489-2492; (c) Cecil, A. R. L.; Hu, Y. L.; Vicent, M. J.; Duncan, R.; Brown, R. C. D., J. Org. Chem. 2004, 69, 3368-3374. (d) Al Hazmi, A. M.; Sheikh, N. S.; Bataille, C. J. R.; Al-Hadedi, A. A. M.; Watkin, S. V.; Luker, T. J.; Camp, N. P.; Brown, R. C. D. Org. Lett. 2014, 16, 5104–5107.
(2). Brown, R. C. D.; Keily, J. F. Angew. Chem. Int. Ed. 2001, 40, 4496.
(3). (a) Cutter, A. C.; Miller, I. R.; Keily, J. F.; Bellingham, R. K.; Light, M. E.; Brown, R. C. D. Org. Lett. 2011, 3, 3988–3991. (b) Watkin, S. V.; Camp, N. P.; Brown, R. C. D. Org. Lett. 2013, 15, 4596–4599. (c) Al-Saffar, F. M.; Brown, R. C. D. Org. Lett. 2017, 19, 3502–3504.
Prof. Richard C. D. Brown
Richard Brown received his first degree (B.Sc. hons) in Chemistry from the University of Southampton in 1990. He stayed in Southampton for postgraduate studies under the guidance of Professor Kocienski F.R.S., obtaining his Ph.D. in 1994 for his thesis “Furan Oxidation Applied to the Synthesis of Salinomycin”. He moved to the University of California Berkeley in 1994 to take up a NATO postdoctoral fellowship in Professor Clayton Heathcock’s research group to work on the syntheses of the alkaloids petrosin C and petrosin D. On his return to the UK in 1996, he took up a six-month sabbatical at Pfizer Central Research before being awarded a Royal Society University Research Fellowship to join the faculty at the University of Southampton. He was promoted to Professor in 2010. His research interests revolve around synthetic organic chemistry and its application, and he is author and co-author of >100 journal and book articles. Current projects include the synthesis of natural products, asymmetric synthesis, and electroorganic synthesis in flow reactors.
